DANGEROUS CHOICES –
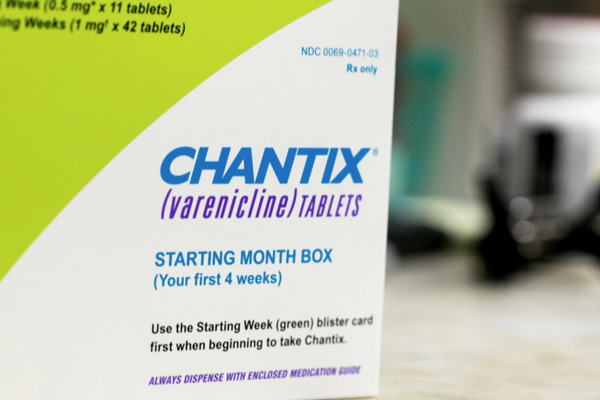
Sept. 18, 2021 – Chantix was approved by the FDA in May 2006 as a prescription medication to help adults aged 18 and over quit smoking and is typically used for 12 to 24 weeks. The recall is for all lots of 0.5 mg and 1 mg varenicline tablets, the Food and Drug Administration said Thursday. Long-term ingestion of the drug can lead to a “potential increased cancer risk in humans, but there is no immediate risk to patients taking this medication.”
For this reason, the FDA said on Friday that patients taking the recalled drug “should continue taking their current medicine until their pharmacist provides a replacement or their doctor prescribes a different treatment.”