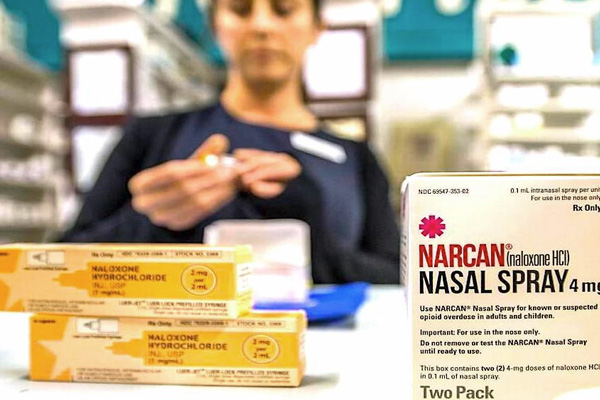
OVERDOSE DRUG OVER THE COUNTER –
Feb. 15, 2023 – The manufacturer, Emergent Biosolutions, said it would revise the packaging and labeling to address those concerns. The FDA will make a final decision on the drug in coming weeks. Panel members urged the FDA to move swiftly rather than waiting for Emergent to conduct a follow-up study with the easier-to-understand label. There’s perhaps a far greater risk of delaying the availability of the product given the climate of this crisis and its devastating consequences,” said Maria Coyle, a pharmacy professor from Ohio State University, who chaired the panel.
The prefilled nasal device, Narcan, is the leading version of the drug in the U.S., which is also available as an injection. If FDA approves, Narcan would be the first opioid treatment to make the regulatory switch to a non-prescription drug.