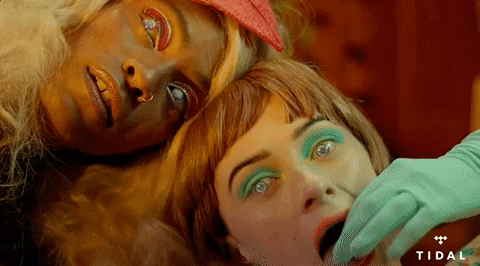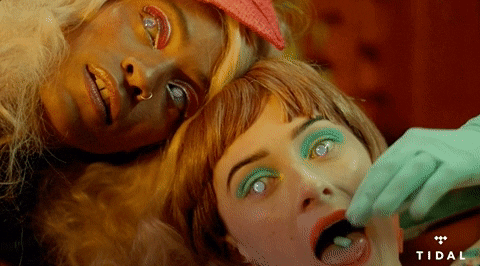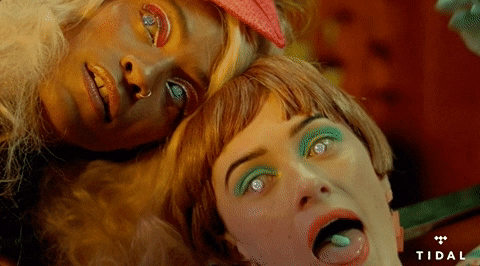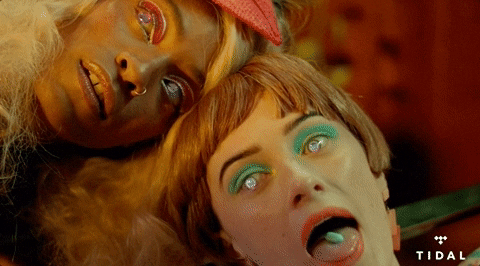GOODBYE YELLOW BRICK ROAD –
June 5, 2024 – The decisions mark a reversal of fortune for MDMA. FDA granted the drug an expedited review process in 2017 based on encouraging early reports about its ability, in combination with talk therapy, to alleviate the PTSD that afflicts millions, including some military veterans. That possibility has been a centerpiece of the recent renaissance of medical research on psychedelic drugs. (MDMA is often termed a psychedelic, though it is less likely to produce some effects, such as visual hallucinations, that are typical for classic psychedelics such as LSD or psilocybin.) Ongoing clinical trials are testing several psychedelic compounds to treat other conditions, including depression and addiction.
In a press release issued after the meeting, Lykos Therapeutics emphasized that FDA is not bound by the committee’s decision. “While we are disappointed in the vote, we are committed to continuing to collaborate with the FDA with their ongoing review of our NDA [new drug application] over the coming weeks,” the release said.