SHALLOW BREATH AND GRAVE –
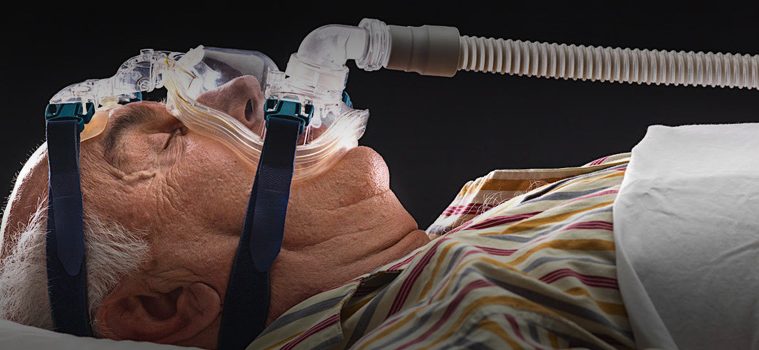
August 17, 2021 – The agency said the possible risks of particulate and chemical exposure from the recalled devices included asthma, skin and respiratory-tract irritation and “toxic and carcinogenic effects” to organs including the kidneys and liver. The Philips recall involved certain BiPAP (bi-level positive air pressure), CPAP (continuous positive air pressure) and ventilator machines manufactured before April 26.
The company has to submit a repair-and-replacement program for the flawed components to the F.D.A. In the meantime, there are shortages and backlogged orders, created by delays in the replacements and the use of similar devices in emergency rooms and intensive-care units during the coronavirus pandemic. Mario Fante, a spokesman for Royal Philips, the parent company of Respironics, estimated that up to two million of the recalled devices were in use in the United States, about half of the global count. These machines are used at home by some of the estimated 24 million Americans with obstructive sleep apnea.