Dysfunctional brain Dopamine caused by Reward Deficiency Syndrome (RDS) – is that when you might just want to get high?
by Kenneth Blum, PhD, DHL, Kai Uwe Lewandrowski, MD, Thomas McLaughlin, MD, Morgan Lorio, MD
April 17 ,2025
Click here and watch a minute or two of B. B. King singing “The Thrill is Gone.” You might notice that he does not look happy. Nor does he sound happy. He says he’s free, but is he?
What happens when the thrill is gone? In fact, what if the thrill was never there to begin with?
The sun is setting on a balmy Saturday night in New York City, and 14-year-old Gerry is all alone sitting on his family’s balcony overlooking the Hudson River being lit up by the mysterious NY Skyline -a wonderful sight! Talking to his friend Pete, living in the same high rise, no novice when it comes to getting high on weed, who is encouraging Gerry to eat that THC delta 9 gummy that Pete gave him in the morning when Gerry angerly said that he was grounded for two weeks because he lied to his mother about a school project. Gerry’s mother Sue, has been married three times whereby Gerry’s biological father died in an auto accident while driving under the influence of alcohol, weed and cocaine. Sue warned Gerry about this since he could remember as young boy. His 19-year-old sister Barbara is facing DUI charges and on her way to rehab. With all this negative information for some unknown reason Gerry for a long time now wanted to get out of his own skin knowing that something was missing. Peter shouted” take it already damn it”!
This troubling scenario has become all too familiar, particularly in the United States, where the opioid crisis has claimed the lives of over a million people. As the nation grapples with a massive wave of addiction, it’s fair to ask: Is there a deeper biological or genetic reason behind the millions now in treatment for substance use disorders? Do we simply point fingers at the Sackler family for the widespread marketing of OxyContin, or are we ready to seek more meaningful explanations?
Since 1995, researchers have proposed a compelling theory involving genetic and neurobiological disruptions in the brain’s reward circuitry. This framework, known as Reward Deficiency Syndrome (RDS), suggests that a malfunctioning reward system contributes to a range of compulsive behaviors—spanning substance abuse, behavioral addictions, and other impulse control disorders. Rather than relying solely on psychological models, RDS offers a neurobiological lens through which we can better understand behaviors such as adolescent drug experimentation and chronic relapse.
So, what exactly is RDS? Who is affected, and how does it develop? Is this condition rooted in our DNA—passed down through generations—or is it shaped by environmental exposures beginning as early as fetal development? Most likely, it’s both. Advances in neuro-epigenetics indicate a complex interplay between inherited traits and environmental influences. The real question is whether we are ready to identify those at risk early on or continue to overlook the underlying causes in a population of over 8 billion people, an estimated 2 billion of whom may carry genetic markers associated with RDS.
Figure 1 Lock-and-Key Principle of Neurotransmitter Binding: This illustration depicts the “lock-and-key” model of ligand-receptor interaction, commonly used to explain how neurotransmitters (ligands) bind to their specific receptor sites in the brain. In this example, properly shaped white keys represent neurotransmitters that fit precisely into the green receptors (locks), triggering a cellular response. The black key, which does not match the receptor’s shape, illustrates a blocked or ineffective binding—representing antagonists or mutated ligand-receptor mismatches. This principle is central to understanding neurotransmission, pharmacologic action, and the neurobiology of reward signaling.
One possible explanation for the widespread presence of RDS is that it once conferred evolutionary advantages. When we engage in rewarding activities—whether substance-related or behavioral—the brain releases dopamine, a neurotransmitter central to pleasure and motivation. This dopamine-driven activity is most prominent in areas like the nucleus accumbens, prefrontal cortex, and motor regions—key components of the brain’s reward network. Receptors in these regions serve as molecular gateways, and when dopamine binds to them, it reinforces behavior. In individuals with RDS, however, this system is impaired, leading to dysfunctional reward processing and a heightened drive to seek stimulation (Figure 1).
The scientific foundation of this field traces back to a landmark 1990 study by Kenneth Blum and Ernest Noble, published in JAMA, which identified the first genetic variant linked to severe alcoholism. At the time, genetic research was still emerging, making this finding a pivotal insight into the biological basis of addiction. Although several dopamine receptor subtypes have since been characterized—currently totaling nine—the D2 receptor earned particular attention for its prominent role in both substance and behavioral dependencies and continues to be a top genetic variant n all metal illness.
The D2 receptor is now recognized as a key modulator in a wide spectrum of behaviors, including cravings for alcohol, cannabis, cocaine, nicotine, and opioids, as well as non-substance-related compulsions such as pathological gambling, eating disorders (including anorexia, bulimia, and binge eating), hoarding, and even issues tied to motivation and attention. Dr. David Smith, in the late 1970s, coined the term “amotivational syndrome” to describe some of these symptoms. Dopamine’s interaction with the D2 receptor is thought to provide inhibitory signals—essentially a “no-go” mechanism—that helps prevent compulsive reward-seeking actions, including excessive drug use or gaming.
In contrast, the D1 receptor plays an excitatory or “go” role in the dopaminergic system. Proper regulation between these pathways is crucial; maintaining dopaminergic homeostasis enables individuals to resist overindulgence. This imbalance may help explain why millions of people in the United States struggle with controlling their use of alcohol or cannabis—even in contexts where substances are legal and widely available (Figure 2).
Figure 2 Dopamine Molecular Structure. Dopamine (C₈H₁₁NO₂), often referred to as the “happiness molecule,” plays a key role in regulating emotional responses, motivation, and the brain’s reward system. It influences how we experience pleasure, desire, and drive. Reproduced in modified form from Blum et al. {Blum, 2016 #8867} Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5103643/figure/F1/
What are the consequences of having an abnormal number of D2 receptors—whether too few or too many? Variations in this receptor are linked to a wide range of compulsive and maladaptive behaviors. These include not only chemical and behavioral addictions but also conditions such as obsessive-compulsive disorder (OCD), eating disorders, post-traumatic stress disorder (PTSD), attention-deficit disorders, and certain expressions of uncontrolled anger. Additionally, the D2 receptor is implicated in the modulation of chronic pain.
The term “Reward Deficiency Syndrome” (RDS), introduced by Kenneth Blum in 1995, describes a genetically influenced disruption in the brain’s reward system that drives compulsive reward-seeking behavior. Now featured in the SAGE Encyclopedia of Abnormal Psychology and referenced in over 1,600 peer-reviewed publications, RDS is defined as:
“A genetically driven impairment in the brain’s reward function that manifests as excessive or abnormal pursuit of pleasure through substances or behaviors such as food, sex, gambling, or digital addiction.”
Genetic research has confirmed that diminished dopamine activity affected by at least seven other neurotransmitters —often due to a specific D2 receptor polymorphism—is present in roughly one-third of the U.S. population. This variant can reduce the availability of D2 receptors by up to 40%, affecting a number of brain functions. The scope of this issue is significant—comparable to the entire population of California (Figure 3).
Figure 3 Prevalence of the DRD2 A1 Allele in the U.S. Population: This infographic illustrates the widespread distribution of the DRD2 A1 gene variant across major ethnic groups in the United States, highlighting its association with Reward Deficiency Syndrome (RDS). Over one-third of the U.S. population—more than 100 million individuals—carry this gene. Prevalence rates are particularly high among Native Americans (85%), Asians (72%), Hispanics (58%), and African Americans (50%). The presence of this gene variant has been linked to a higher risk of addictive behaviors and impaired dopamine regulation, underscoring the importance of personalized approaches to addiction treatment and prevention.
This group of conditions encompasses a wide range of neuropsychiatric and behavioral disorders, including post-traumatic stress disorder (PTSD), attention deficit hyperactivity disorder (ADHD), motor and vocal tics, Tourette’s, autism spectrum disorders, obsessive-compulsive tendencies, compulsive sexual behavior, and disordered eating patterns such as binge eating. A clearer understanding of their interconnection emerges when viewed through the lens of shared genetic influences and overlapping neurobiological mechanisms (Figure 4).
Figure 4 Visualizing Reward Deficiency Syndrome (RDS): This illustration symbolizes the dynamic interplay between the human brain and its external environment, capturing the essence of Reward Deficiency Syndrome (RDS). RDS is a neurogenetic condition marked by impaired dopamine signaling, often due to genetic variants such as the DRD2 A1 allele. Individuals affected by RDS may experience reduced activation of the brain’s reward circuitry, particularly within the nucleus accumbens and prefrontal cortex, leading to an increased vulnerability to compulsive behaviors and substance use. The radiant, outward-reaching energy beams represent the untapped potential for creativity, motivation, and emotional regulation, which can remain suppressed when dopamine pathways are dysregulated. This image also echoes the importance of conscious lifestyle choices—such as nutrition, mindfulness, and spiritual practices—in reshaping brain chemistry and unlocking deeper well-being, as inspired by the quote from Leonard Perlmutter.
Being born with a specific genetic marker—namely, a variant of the DRD2 gene—can increase susceptibility to a wide range of conditions that fall under the umbrella of Reward Deficiency Syndrome (RDS). These include attention-related issues like ADHD, anxiety-based disorders such as OCD and PTSD, and a variety of addictive behaviors, including substance abuse (e.g., opioids, high-potency THC products, alcohol), as well as non-substance addictions like gambling. This predisposition is tied to a decrease in dopamine receptor density (particularly D1 through D5), which can make individuals more vulnerable to compulsive behaviors and emotional dysregulation.
The origin of this vulnerability lies in the DRD2 gene, which plays a key role during fetal development by regulating the formation of D2 receptors in the brain. A variant known as the A1 allele leads to fewer receptors, compromising the brain’s reward response mechanisms. Extensive research supports the idea that addiction can run in families, passed from parents to children through this same genetic pathway. This finding strongly supports the role of inherited biology—Nature—in addictive risk. That said, environmental influences—Nurture—also matter.
This brings us to the field of epigenetics, which explores how life experiences and external conditions can influence gene expression. So, while someone may inherit the genetic blueprint for addiction, environmental triggers can significantly shape the final outcome (Figure 5).
Figure 5 Epigenetics – Beyond the DNA Sequence: This illustration depicts the intricate interplay between genetic material and neural signaling, symbolizing the role of epigenetics in shaping individual identity. While our DNA provides a foundational blueprint, epigenetic mechanisms—such as methylation, histone modification, and environmental influences—can alter gene expression without changing the genetic code itself. These modifications can be influenced by behavior, stress, nutrition, and trauma, and in some cases, may be passed on to future generations. The image highlights how experiences can impact who we are, offering insight into the question: What makes me “me”?
Is DNA Our Destiny? The notion that our DNA determines everything about us—our destiny—has captivated scientists and the public alike. With the rise of genetic science in the 20th century and the rapid advances in biotechnology, many hoped that unlocking the genome would unravel the secrets of identity. Indeed, these efforts led to discoveries explaining traits like eye color, the origins of conditions such as albinism, and the hereditary nature of diseases like cystic fibrosis. One milestone in this journey was the Human Genome Project, a decade-long endeavor that decoded more than 3 billion base pairs—the building blocks that define our species and individual uniqueness whereby only about 8% actually have important functioning, especially as it relates to mental health. .
However, over the past ten years, the initial excitement has given way to deeper reflection. Scientists have begun to recognize that identifying genes associated with diseases and behaviors only scratches the surface of human complexity. A compelling question arises: how can identical twins, sharing the same genetic blueprint, differ in appearance, health, or personality? The answer lies not solely in DNA, but in a parallel realm of biology—epigenetics.
Epigenetics explores heritable changes in gene activity that occur without alterations to the DNA sequence itself. These modifications can be passed both within and across generations. Consider the case of bees: despite sharing identical genomes, some larvae become worker bees while others become queens. This divergence stems from diet—larvae fed pollen develop into workers, while those nourished with royal jelly mature into queens. The same genes, vastly different outcomes.
Such biological plasticity is governed by epigenetic mechanisms. One key process is DNA methylation, where chemical tags attach to DNA bases (adenine, thymine, cytosine, and guanine), influencing gene expression. Another critical system involves histone modification. Histones are proteins that package DNA and maintain its structural integrity. When chemical makers are added to histones, they can loosen or tighten the DNA coil, thereby regulating access to genetic instructions. For example, methylation near the DRD2 gene can reduce the number of dopamine D2 receptors in the brain, impacting behavior and reward processing. Together, these epigenetic insights offer a more dynamic view of human biology—where environment, lifestyle, and even prenatal conditions interact with our genes to shape who we are.
In 2007, a groundbreaking discovery in epigenetics challenged long-standing assumptions in biology. Researchers working with mice carrying the Agouti gene—a variant associated with yellow fur, obesity, diabetes, and shortened lifespan—introduced a dietary intervention rich in methyl donors, compounds known to influence gene expression. The results were astonishing: offspring from these mice were born lean, brown, free of metabolic illness, and lived significantly longer. Though the Agouti gene remained in their genome, it had been epigenetically silenced through methylation. Even more remarkable, these beneficial traits were passed on to the subsequent generation.
This revelation led to a profound insight: health and disease risks may reflect not only our parents’ behaviors, but also those of our grandparents. Factors such as nutrition, exercise, stress, and emotional care—especially in early life—can shape how genes are expressed across generations. In essence, DNA lays the foundation, but epigenetics scripts the performance. The well-known phrase “Genetics loads the gun, but epigenetics pulls the trigger” captures this shift in understanding. An early 70s study by Blum’s group one of the first investigations of epigenetic effects showed that prenatal administration of THC resulted in generational enhanced morphine sensitivity.
Supporting this, a study from Yasmin Hurd’s team at Mount Sinai in New York revealed that mice injected with purified THC not only exhibited changes in brain chemistry, but their grand-offspring had reduced D2 receptor density and a heightened tendency to self-administer heroin—clear evidence that drug exposure in one generation can alter neurobiology and behavior in the next.
What does this mean for humans? Some people inherit two copies of the A1 variant of the DRD2 gene, leading to a substantial reduction—up to 40%—in dopamine D2 receptors in reward-related brain regions such as the nucleus accumbens. Functional imaging has shown that these individuals exhibit dampened responses to natural rewards. In one study by Eric Stice and colleagues, published in Neuroscience, carriers of the A1 allele experienced a blunted neural response to stimuli like a milkshake, compared to those with the typical DRD2 variant. This muted activation may underlie behaviors like overeating, as the usual pleasures fall short of satisfaction, driving excessive consumption to chase fleeting gratification.
This flattening of the reward system—the very circuitry that fuels motivation and joy—can leave life feeling muted. As B.B. King sang, “The thrill is gone.” But perhaps, for some, the thrill was never truly there to begin with. This may explain deep-seated restlessness or DNA- pre-addictive vulnerability observed across generations.
For those born with fewer dopamine receptors, typical pleasures might not suffice. To compensate, many seek out intensely stimulating experiences—drugs, alcohol, risky behaviors, gambling, or extreme sports—as a way to break through the neurochemical monotony. This is the essence of Reward Deficiency Syndrome (RDS): a biological predisposition to under-react to everyday rewards, leading to compulsive behaviors in search of fulfillment.
In sum, both genetic inheritance and environmental exposure shape our neurological landscapes. And for those wired with dopamine deficits, the quest for excitement may not just be a desire—it may feel like a necessity.
Could this narrative reflect the essence of human behavior? While numerous questions remain and further scientific investigation is warranted, the evidence increasingly suggests that Reward Deficiency Syndrome (RDS) pushes individuals to seek stronger stimuli in an effort to feel something—anything—approaching normalcy. This pursuit of a heightened experience aligns with the natural human desire for the dopaminergic surge that accompanies excitement and reward.
It’s why, for some, a roller coaster ride or a bite of chocolate delivers joy, while for others, such experiences feel flat or unremarkable—echoing B.B. King’s iconic line, “The thrill is gone.” Much of life’s pleasure stems not only from the event itself but from the anticipation leading up to it. However, in those affected by RDS, that motivational spark—the drive to approach rewarding experiences—is significantly dulled.
This plays out in real lives, such as those of Pete and Gerry, whose personal struggles may be compounded by both genetic predispositions and neurological development. Their prefrontal cortexes, like in many adolescents, remain under-myelinated, impairing executive functions such as impulse control and long-term decision-making. Even without genetic risk, the adolescent brain—wired for novelty and thrill—exhibits high dopaminergic activity. This biological sensitivity helps explain why substances like cannabis or alcohol can feel particularly potent and rewarding during the teenage years. Unfortunately, this natural tendency can steer some toward danger, regardless of genetic makeup.
Given these risks, early identification of at-risk individuals—starting as early as birth—could prove invaluable. Testing for genetic vulnerability does not equate to labeling a child an addict; rather, it empowers parents and caregivers with foresight in a world increasingly saturated with hazardous temptations, including fentanyl-laced opioids that continue to claim lives across the United States .Most recently, a paper by Alireza Sharafshah and others, provided strong evidence from a meta -analysis using very large data banks unequivocally revealed that the Genetic Addiction Risk Severity (GARS®) test was an accurate predictor of preaddiction.
In Psychology Today, author Mark Lewis reflected on this phenomenon in his 2013 piece titled “The Thrill is Gone,” pondering whether RDS could partially explain the relentless—and sometimes deadly—pursuit of psychoactive highs. For B.B. King, the thrill once existed but faded. For others, it may never have arrived in the first place. That aching void can be the silent fuel behind compulsive behavior.
Despite this sobering reality, there is reason for optimism. Our dedicated team of researchers and clinicians has spent decades exploring interventions. One major innovation is the GARS test, a patented panel that analyzes ten genes involved in the brain’s reward system, including dopamine, serotonin, endorphins, GABA, glutamate, and endocannabinoids. With 103 peer-reviewed publications supporting its utility, GARS represents a powerful tool for identifying neurogenetic vulnerability early on.
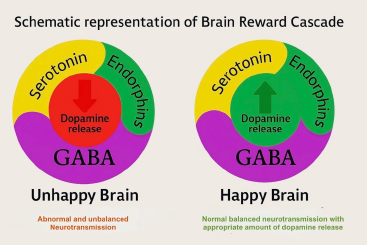
Figure 6. Schematic Representation of the Brain Reward Cascade This diagram compares neurotransmitter dynamics in two distinct states of brain reward function.
(Left) Unhappy Brain: Depicts disrupted neurochemical signaling with excessive GABA activity and diminished dopamine release, often associated with Reward Deficiency Syndrome (RDS).
(Right) Happy Brain: Illustrates a balanced cascade where serotonin, endorphins, and GABA interact harmoniously to facilitate healthy dopamine release in the reward center, resulting in a stable sense of pleasure, motivation, and well-being.
Complementing this diagnostic tool is a nutraceutical solution known as KB220, first developed in 1976. Neuroimaging studies have shown that KB220 supports “dopamine homeostasis,” even in abstinent heroin-dependent patients. With more than 33 clinical trials—and additional preclinical studies—demonstrating efficacy and mechanisms of action, KB220 offers a path toward restoring balance in disrupted reward systems.
The core idea behind this intervention is simple yet profound: to cultivate a “Happy Brain”—a neurochemically balanced state where dopamine is released in optimal, steady amounts at key reward centers like the nucleus accumbens. This approach has the potential to transform what begins, even at birth, as an “unhappy brain” into one capable of experiencing sustained emotional well-being (Figure 6).
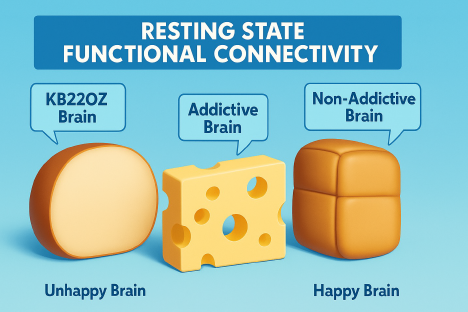
Recently, neuroimaging has revealed that diminished resting-state functional connectivity (rsFC) may contribute to the development of addictive behaviors. Typically, rsFC reflects healthy “cross talk” between brain regions—such as the hippocampus (memory), nucleus accumbens (craving), and cingulate gyrus (decision-making)—that collaborate to regulate impulse control and motivation. When this communication network is weakened, the brain’s regulatory capacity is impaired, increasing vulnerability to addiction. This disrupted pattern is conceptually illustrated using the Swiss cheese model (Figure 7)
Figure 7 Cheese illustrations depict levels of brain connectivity. The Addictive Brain, characterized by gaps in communication between regions, is symbolized by hole-filled cheese—indicating disrupted resting-state connectivity. In contrast, the Non-Addictive Brain shows intact signaling pathways with minimal disruptions. Supplementation with KB220Z has been shown to enhance these neural connections, supporting improved executive function and decision-making.
Published research from our team demonstrated that in non-addicted rats, KB220Z enhanced functional connectivity across several key brain regions when compared to placebo. These activated areas—such as the prelimbic and infralimbic cortices, nucleus accumbens, cingulate gyrus, anterior thalamic nuclei, and hippocampus—are all involved in memory, craving regulation, and executive decision-making. While negative epigenetic influences like drug exposure and chronic stress can diminish resting-state functional connectivity (rsFC), positive interventions such as KB220 may promote beneficial epigenetic modulation by increasing rsFC.
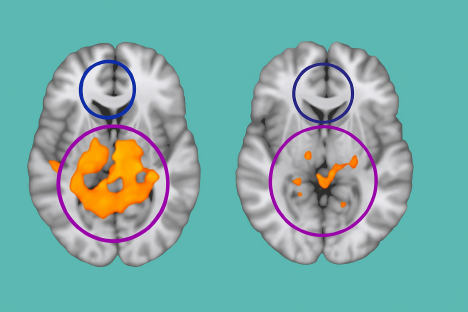
Most notably, a placebo-controlled crossover study involving abstinent heroin users revealed that KB220Z significantly enhanced rsFC. Within just one hour of oral administration, dopaminergic circuits became more active, excessive emotional responses linked to cerebellar hyperactivity were reduced, and rsFC patterns were normalized—providing a glimpse into what balanced dopamine transmission may look like (Figure 8).
Figure 8 Resting-state fMRI brain scans taken one hour after a single oral dose of KB220Z. The left image represents the placebo condition, showing limited activation and reduced functional connectivity across reward-related brain regions. The right image illustrates enhanced neural activity following KB220Z administration, with increased resting-state functional connectivity notably in the dopaminergic pathways. These changes suggest that KB220Z may support dopamine homeostasis by improving communication between brain regions involved in reward, motivation, and emotional regulation.
Much work remains ahead—including the exploration of gene editing—but we are finally asking the right questions. The general public is increasingly aware of the growing body of international research supporting the concept of Reward Deficiency Syndrome (RDS), which encompasses both genetic predispositions present from birth and environmental or epigenetic influences passed through generations. Although the definitive origins of RDS are still under investigation—including genetic, psychological, and even spiritual factors such as the emerging notion of “Geneospirituality”—progress is undeniable. Our scientific institutions, particularly those supported by government funding, are advancing multidisciplinary research at an impressive pace.
As Dr. Nora Volkow emphasized, addressing this crisis requires “ALL HANDS ON DECK!” Millions of individuals continue to struggle daily with their inability to experience natural pleasure or a sense of well-being—often turning to substances in a desperate attempt to feel “normal.” For some, the consequences are severe: incarceration for minor offenses, such as marijuana possession, although recent reforms and pardons are offering a glimmer of justice. Yet the fundamental question remains: is there a lasting solution to the genetically influenced and environmentally exacerbated dopamine dysfunction seen in RDS? Encouragingly, emerging evidence suggests that epigenetic shifts—heritable and potentially reversible—may hold the key. Is it indeed as simple as considering a potential “out of the box” thinking we call RDS-solution system (RDSS). In this scenario, firstly at entry a patient completes the validated RDSQ29 questionnaire ; then the patient following this firsts step “medical necessity provided a cheek cell sample for genetic DNA testing utilizing the GARS and RNA profiling to measure brain circuitry proteins ; Thirdly, based on the GARS results the patient receives a gene based personalized KB220; fourthly, going forward in the treatment process continued RNA profiling objectively checks for the reoccurrence of so called normal neurotransmitter (including dopamine) integrity.
With the unwavering efforts of scientists around the world, we may one day realize a future free from chronic emotional pain, where human potential is fully expressed, and suffering is replaced by balance and inner peace—perhaps even a return to the harmony once imagined in the Garden of Eden.